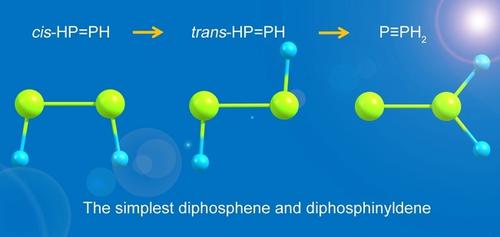
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common, but their properties have theoretical importance.
Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by large steric hindrance. In general, diphosphenes react like alkenes.
History
In 1877, Köhler and Michaelis claimed what would have been the first isolated diphosphene (PhP=PPh), The structure of Köhler and Michaelis' product was later revised. and X-ray crystallographic analysis proved that this "diphosphene" only had P-P single bonds and was in fact primarily a four-membered ring of the form (PPh)4. The isolation of phosphorus ylide and phosphaalkenes suggested that compounds with P=P bonds could be made.
Yoshifuji et al's isolated a sterically-hindered diphosphene in 1981. That compound's P-P bond distance is 2.034 Å, which is much shorter than the average bond length in (C6H5P)5 (2.217 Å) and (C6H5P)6 (2.237 Å) and indicates double-bond character.
Synthesis
Following Maasaka Yoshifuji and his coworkers' 1981 preparation of bis(2,4,6-tri-tert-butylphenyl)diphosphene, most disphosphene syntheses involve dehalogenation of bulkyl aryldichlorophosphine (ArPCl2). Mg is a typical dehalogenation reagent:
- 2 ArPCl2 2 Mg → ArP=PAr 2 MgCl2
Such a synthesis works also for trisalkylsilylphosphines, or N-heterocyclic boro-phosphines.
Ylide-stabilized diphosphenes
Examples of di-vinyl-substituted diphosphenes arise via a ring opening/dimerization process from kinetically unstable 2H-phosphirenes. However, the conjugation caused the compounds to exhibit reactivity closer to a phosphinidene.
Structure
Cyclic voltammetry and UV/Vis spectra indicate that boryl-substituted diphosphenes have lower LUMO level and larger HOMO-LUMO gap than aryl-substituted diphosphenes.
Geometry
According to X-ray crystallography, the following parameters describe bis(2,4,6-tri-tert-butylphenyl)diphosphene: P-P = 2.034 (2) Å; P-C = 1.826 (2) Å; P-P-C = 102.8 (1)o; C-P-P-C = 172.2 (1)o. Compared with the length of a P-P single bond in H2PPH2 (2.238 Å), the P-P bond distance is much shorter, which reveals double bond character. The trans orientation is the thermodynamically preferred isomer.
Spectroscopic properties
Diphosphene compounds usually exhibit a symmetry-allowed () (intense) and symmetry-forbidden () (weak) electronic transitions. In the Raman spectrum, the P=P vibration is enhanced by resonance with allowed the transition than with the forbidden transition due to different geometries of excited states and enhancement mechanism. Also the observed strong Raman shifts for (CH(SiMe
3)
2)
2P
2and (CH(SiMe3)2P=PC(SiMe3)2) suggest stronger dipnictenes feature of diphosphene compared with P-P single bond.
Reactivity
Lithium aluminium hydride reduces diphosphene to give diphosphanes.
Carbenes add across the double bond, to give diphosphiranes, which further rearrange to 1,3-diphospha-allenes in strong bases.
Diphosphene is inert to oxygen but cycloadds to ozone to give highly unstable phosphorus-oxygen rings that tend to attack the phosphorus' organyl substituents. The reaction with ozone is much more rapid and indicates a 2:1 (ozone:diphosphene) stoichiometry.
When treated with strongly nucleophilic NHC's, the P=P bond cleaves giving phosphinidene compounds:
- RP=PR L → 2 RP−L
Coordination to transition metals
Diphosphines form a variety of [[coordination complexes.. Diphosphenes can bind to transition metal either in a η1 or in a η2 mode.
[{{chem2|Fe(CO)4[P2(CH(SiMe3)2}) is obtained by treating Na2[Fe(CO)4] with dichlorobis(trimethylsilyl)methylphosphine. The related complex [ArP=PAr]Fe(CO)4 (Ar=2,4,6-tri-tert-butylphenyl) arises by treating diphosephene with Fe2(CO)9.
η2-coordination is illustrated by (M(PhP=PPh)L2) (with M=Pt or Pd and L = (PPh3)2 or Ph2P[CH2]2PPh2).
See also
- Diazene
- Double bond rule
References